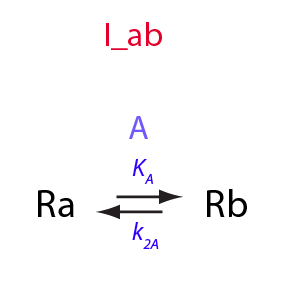
Derivation of equations for I_ab model
Simple intramolecular isomerization

2. Derivation of a main equation
3. Define functions for equilibrium concentrations
4. Test if solution is meaningful
5. Check whether the solution satisfies all initial equation and conditions
6. Save results on disk for future use
In this notebook I will formally derive equations for the simplest isomerization equilibrium
Analysis will be done in I_ab_analysis.mn
clean up workspace
reset()
Set path to save results into:
ProjectName:="I_ab";
CurrentPath:="/Users/kovrigin/Documents/Workspace/Global Analysis/IDAP/Mathematical_models/Equilibrium_thermodynamic_models/I_ab/";
![]()

Isomerization constant
These relationships serve as restraints for solve(), but not restrict these values in calculations!
K_A
K_A ;
assume(K_A > 0):
assumeAlso(K_A , R_):
![]()
Total concentrations
Rtot - total concentration of the molecule
Rtot;
assumeAlso(Rtot>0):
assumeAlso(Rtot,R_):
![]()
Common equilibrium concentrations
Raeq - equilibrium concentration of Ra form
Raeq;
assumeAlso(Raeq>0):
assumeAlso(Raeq<Rtot):
assumeAlso(Raeq,R_):
![]()
Rbeq - equilibrium concentration of Rb form
Rbeq;
assumeAlso(Rbeq>0):
assumeAlso(Rbeq<Rtot):
assumeAlso(Rbeq,R_):
![]()
anames(Properties,User);
![]()
2. Derivation of a main equation
Total concentrations of the molecule
eq2_1:= Rtot = Raeq + Rbeq
![]()
Write equilibrium thermodynamics equations
eq2_2:= K_A = Rbeq / Raeq
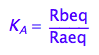
Express Raeq as a function of all constants and total concentrations
Express Rbeq
eq2_2;
solve(%,Rbeq);
%[1][1];
eq2_3:= Rbeq= %
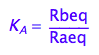
![piecewise([K_A*Raeq in Dom::Interval(0, Rtot), {K_A*Raeq}], [not K_A*Raeq in Dom::Interval(0, Rtot), {}])](I_ab_derivation_images/math10.png)
![]()
![]()
Substitute to the mass balance equation
eq2_1;
% | eq2_3;
eq2_4:= %:
![]()
![]()
Express Raeq
eq2_4;
solve(%, Raeq);
eq2_5:= Raeq=%[1]
![]()

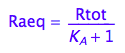
Express Rbeq
eq2_3;
eq2_6:= % | eq2_5;
![]()
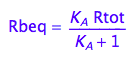
Summary of equations for all species
Independent parameters:
Rtot, K_A
![]()
[Ra]
eq2_5
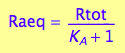
[Rb]
eq2_6
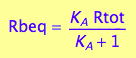
Generate functions
fRaeq:= (Rtot, K_A) --> eq2_5[2]
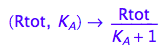
fRbeq:= (Rtot, K_A) --> eq2_6[2]
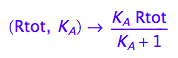
4. Test if solution is meaningful
Set some realistic values for constants:
Total_R:=1;
Ka:=0.5;
![]()
![]()
Test that all equilibrium concentrations are positive values:
fRaeq(Total_R, Ka);
fRbeq(Total_R, Ka);
if (fRaeq(Total_R, Ka)>0 and
fRbeq(Total_R, Ka)>0
)
then
print(Unquoted,"Solution is meaningful.");
else
print(Unquoted,"WARNING!!!!");
print(Unquoted,"Solution is NOT meaningful: some concentrations become negative!");
end_if
![]()
![]()
Solution is meaningful.
5. Check whether the solution satisfies all initial equation and conditions
Here are all original independent
equations:
eq2_1;eq2_2;
![]()
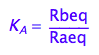
Test eq2_1
eq2_1;
% | eq2_5 | eq2_6;
normal(%);
bool(%)
![]()
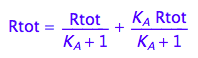
![]()
![]()
The found solution satisfies all original equations
5. Save results on disk for future use
(you can retrieve them later by executing: fread(filename,Quiet))
ProjectName
![]()
Eq_Raeq_I_ab:= eq2_5;
Eq_Rbeq_I_ab:= eq2_6;
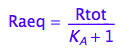
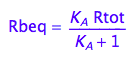
Reassign function names
fRaeq_I_ab:=fRaeq:
fRbeq_I_ab:=fRbeq:
filename:=CurrentPath.ProjectName.".mb";
write(filename,Eq_Raeq_I_ab,Eq_Rbeq_I_ab, fRaeq_I_ab, fRbeq_I_ab)

Conclusions
1. I derived analytical solutions for the I_ab system