Testing the I_ab model
The I_ab_T model is the simplest two-state isomerization model with the temperature dependence
Contents
close all clear all
Here you will find all figures
figures_folder='I_ab_T_testing_figures';
Test Computation of Equilibrium Concentrations
Set some meaningful parameters
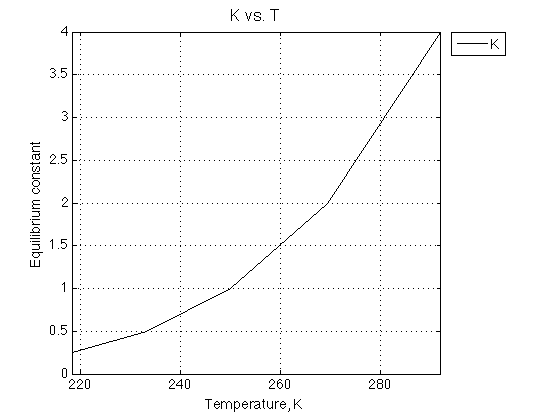
R_gas=8.314510; % Gas constant J /mol /K Rtotal=1; % Receptor concentration, M % these are the constants we want to create K_array=[0.25 0.5 1 2 4 ]; dH= 20000; % J/mol dS= 80; % J/mol/K T_array=[]; for counter=1:length(K_array) % compute temperature T= dH./(dS-R_gas*log(K_array(counter))); T_array = [T_array ; T]; end figure_handle=equilibrium_thermodynamic_equations.plot_populations(... T_array, K_array, {'K'}, 'K vs. T', ... [min(T_array) max(T_array) ], [0 max(K_array) ]); xlabel('Temperature, K','FontSize',14); ylabel('Equilibrium constant','FontSize',14); % save it results_output.output_figure(figure_handle, figures_folder, 'K_plot');
Set appropriate options for the model (see model file for details)
model_numeric_solver='analytical' ; model_numeric_options='n/a'; concentrations_array=[]; for counter=1:length(T_array) % compute [concentrations species_names] = equilibrium_thermodynamic_equations.I_ab_T_model(... Rtotal, T_array(counter), dH, dS,... model_numeric_solver, model_numeric_options); % collect concentrations_array = [concentrations_array ; concentrations]; end
Plot
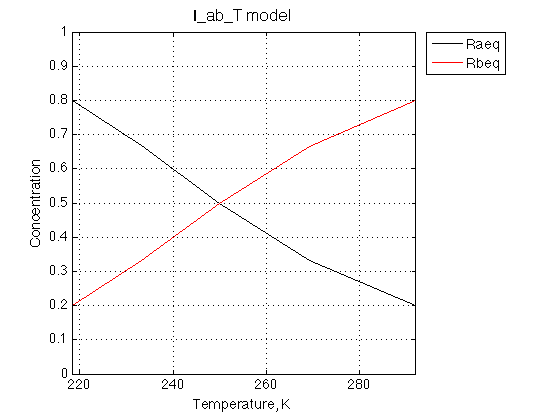
Figure_title= 'I_ab_T model'; X_range=[min(T_array) max(T_array)+0.1 ]; % extend X just a bit past last point Y_range=[0 Rtotal ]; % keep automatic scaling for Y % display figure figure_handle=equilibrium_thermodynamic_equations.plot_populations(... T_array, concentrations_array, species_names, Figure_title, X_range, Y_range); xlabel('Temperature, K','FontSize',14); % save it results_output.output_figure(figure_handle, figures_folder, 'Concentrations_plot');
Observations
The ratio between Ra and Rb forms changes appropriately with K_A value.
Conclusion
The model equilibrium_thermodynamic_equations.I_ab_model() works well.