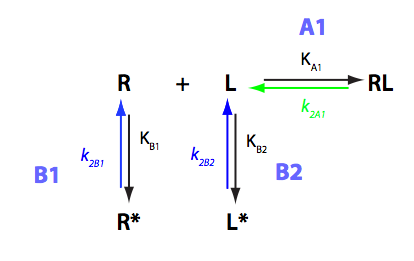
Derivation of equations for U-R-L model
Binding coupled with intramolecular isomerization of both receptor and a ligand into unbinding conformations

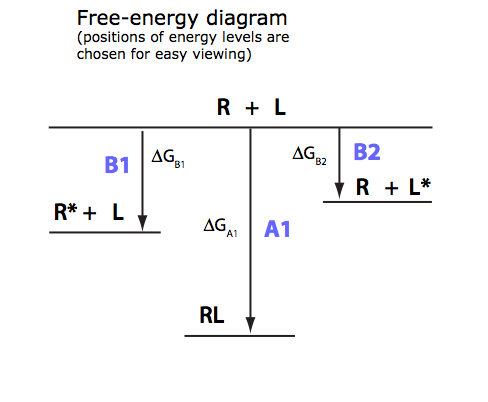

2. Derivation of a main equation
3. Define functions for equilibrium concentrations
6. Save results on disk for future use
In this notebook I will derive analytical solutions for U-R-L model and prepare equations for numerical simulations.
clean up workspace
reset()
Set path to save results into:
ProjectName:="U_R_L";
CurrentPath:="/Users/kovrigin/Documents/Workspace/Data/Data.XV/EKM16.Analysis_of_multistep_kinetic_mechanisms/Equilibria/U_R_L_model/";
![]()
![]()
Binding and isomerization constants
All binding constants I am using are association constants.
These relationships serve as restraints for solve(), but not restrict these values in calculations!
K_A_1
K_A_1 ;
assumeAlso(K_A_1 > 0):
assumeAlso(K_A_1 , R_):
![]()
Isomerization constants are formation constants for the alternative species
K_B_1
K_B_1 ;
assumeAlso(K_B_1 > 0):
assumeAlso(K_B_1 , R_):
![]()
K_B_2
K_B_2 ;
assumeAlso(K_B_2 > 0):
assumeAlso(K_B_2 , R_):
![]()
Total concentrations
Rtot - total concentration of the receptor
Rtot;
assumeAlso(Rtot>0):
assumeAlso(Rtot,R_):
![]()
Ltot - total concentration of a ligand
Ltot;
assumeAlso(Ltot>0):
assumeAlso(Ltot,R_):
![]()
Common equilibrium concentrations
Req - equilibrium concentration of a receptor monomer
Req;
assumeAlso(Req>0):
assumeAlso(Req<Rtot):
assumeAlso(Req,R_):
![]()
Rstareq - equilibrium concentration of an nonbinding receptor isomer
Rstareq;
assumeAlso(Rstareq>0):
assumeAlso(Rstareq<Rtot):
assumeAlso(Rstareq,R_):
![]()
Leq - equilibrium concentration of a free ligand
Leq;
assumeAlso(Leq>0):
assumeAlso(Leq<Ltot):
assumeAlso(Leq,R_):
![]()
Lstareq - equilibrium concentration of a nonbinding ligand isomer
Lstareq;
assumeAlso(Lstareq>0):
assumeAlso(Lstareq<Ltot):
assumeAlso(Lstareq,R_):
![]()
RLeq - equilibrium concentration of a receptor-ligand complex
RLeq;
assumeAlso(RLeq>0):
assumeAlso(RLeq<Rtot):
assumeAlso(RLeq,R_):
![]()
anames(Properties,User);

2. Derivation of a main equation
Working equation: I will try to express analytical [L] from equation for a total concentration of a receptor or use the expression for a numeric solution if analytical is not possible
eq2_1:= Rtot= Req + Rstareq + RLeq;
eq2_2:= Ltot= Leq + Lstareq + RLeq;
![]()
![]()
Equilbrium thermodynamic equations:
eq2_3:= K_A_1 = RLeq/(Req*Leq)
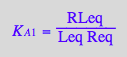
eq2_4:= K_B_1 = Rstareq/Req
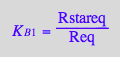
eq2_5:= K_B_2 = Lstareq/Leq
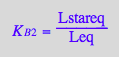
Express all species in terms of Req and Leq
Lstareq
eq2_5;
solve(%, Lstareq);
%[1][1];
eq2_6:= Lstareq = %
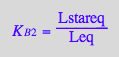
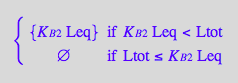
![]()
![]()
Rstareq
eq2_4;
solve(%,Rstareq);
%[1][1];
eq2_7:= Rstareq = %
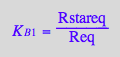

![]()
![]()
RLeq
eq2_3;
solve(%,RLeq);
%[1][1];
eq2_8:= RLeq = %
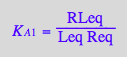

![]()
![]()
Substitute into mass action laws
express Req
eq2_2;
% | eq2_6 | eq2_8;
solve(%,Req);
%[1][1];
eq2_9:= Req = %
![]()
![]()
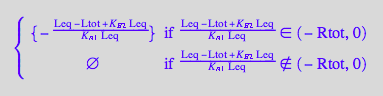
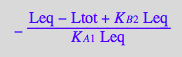
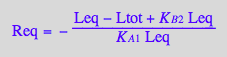
eq2_1;
% | eq2_8 | eq2_7 | eq2_9 ;
eq2_10:= %:
![]()
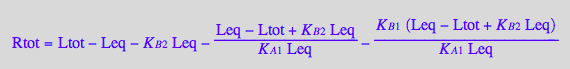
Solve for Leq
solutions2_10:=solve(eq2_10,Leq)

Extract solutions
eq2_11:= solutions2_10[i,1] $ i=1..nops(solutions2_10);
nops(%)
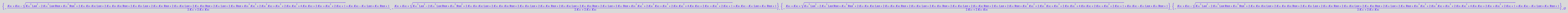
![]()
Is the 1st solution a combination of 2nd and 3rd?
solution1:=eq2_11[1]; // a sequence of roots
solution2:=eq2_11[2][1]; // extract equation out of a sequence
solution3:=eq2_11[3][1]; // extract equation out of a sequence
if solution2 in solution1
then print(Unquoted,"First set of roots contains the second root.");
else print(Unquoted,"First set of roots DOES NOT contain the second root!");
end_if;
if solution3 in solution1
then print(Unquoted,"First set of roots contains the third root.");
else print(Unquoted,"First set of roots DOES NOT contain the third root!");
end_if;
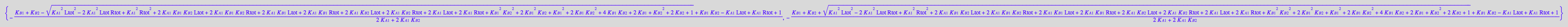


First set of roots contains the second root.
First set of roots contains the third root.
Check correctness of the solutions by substitution into original equation solved:
Check the first root
test1:= eq2_10 | Leq=solution2:
normal(%);
![]()
Check the second root
test1:= eq2_10 | Leq=solution3:
normal(%);
![]()
Both solutions are correct.
Select meaningful solution by substituting parameters
solution2 | K_A_1=1 | K_B_1=1 | K_B_2=1 | Ltot=1 | Rtot =1;
float(%);
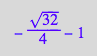
![]()
Not meaningful.
solution3 | K_A_1=1 | K_B_1=1 | K_B_2=1 | Ltot=1 | Rtot =1;
float(%);
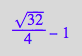
![]()
Solution 3 is meaningful!
Assign it to an equation
eq2_12:= Leq = solution3
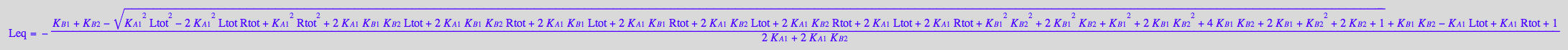
Derive apparent constant value
If only total unbound R and total unbound L is measured what is the apparent binding constant?
eq2_13:= R_U=Req+Rstareq;
eq2_14:= L_U=Leq+Lstareq;
![]()
![]()
Apparent binding constant in terms of 'unbound species'
eq2_15:= Kapp= RLeq/(R_U*L_U);
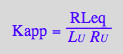
Express in terms of microscopic species:
eq2_16:= eq2_15 | eq2_13 | eq2_14

Express in terms of true constants
eq2_16 | eq2_6 | eq2_7 | eq2_8;
normal(%);
eq2_17:=%;
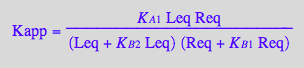
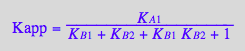
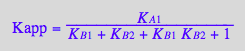
Factor the denominator
op(eq2_17,[2,2,1]);
factor(%);
eq2_18:= %
![]()
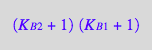
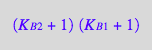
Reassemble the equation
op(eq2_17,[1]) = op(eq2_17,[2,1])/eq2_18;
eq2_19:= %:
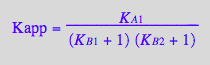
Summary of equations for equilibrium concentrations
Rtot= f(Leq, Leq and constants)
eq2_10
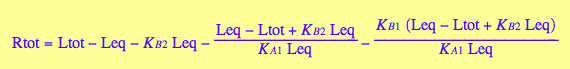
Analytical solution for [L]:
eq2_12

[R]
eq2_9

[L*]
eq2_6
![]()
[R*]
eq2_7
![]()
[RL]
eq2_8
![]()
Apparent binding constant (using full concentrations of unbound species)
eq2_19
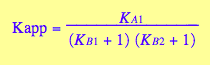
3. Define functions for equilibrium concentrations
Define functions for plotting and analysis
[L]
fLeq_U_R_L:= (Rtot, Ltot, K_A_1, K_B_1, K_B_2) --> eq2_12[2]

[R]
fReq_U_R_L:= (Rtot, Ltot, K_A_1, K_B_1, K_B_2) --> eq2_9[2] | eq2_12
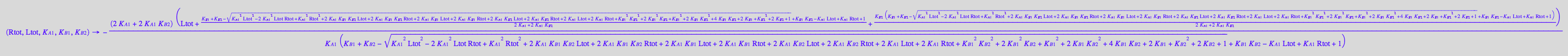
[R*]
fRstareq_U_R_L:= (Rtot, Ltot, K_A_1, K_B_1, K_B_2) --> eq2_7[2] | eq2_9 | eq2_12
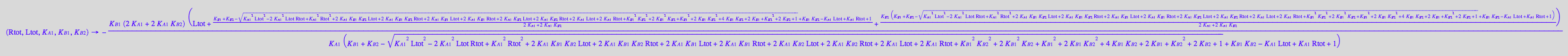
[L*]
fLstareq_U_R_L:= (Rtot, Ltot, K_A_1, K_B_1, K_B_2) --> eq2_6[2] | eq2_12
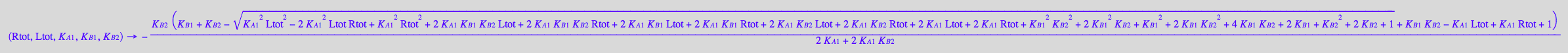
[RL]
fRLeq_U_R_L:= (Rtot, Ltot, K_A_1, K_B_1, K_B_2) --> eq2_8[2] | eq2_9 | eq2_12
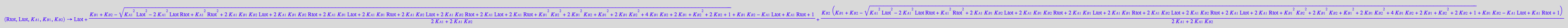
8. Save results on disk for future use
(you can retrieve them later by executing: fread(filename,Quiet))
ProjectName
![]()
Make "portable" equations (unique names for future use):
Rtot_U_R_L:=eq2_10;
Leq_U_R_L:=eq2_12;
Req_U_R_L:=eq2_9;
Lstareq_U_R_L:=eq2_6;
Rstareq_U_R_L:=eq2_7;
RLeq_U_R_L:=eq2_8;
Kapp_U_R_L:= eq2_19;
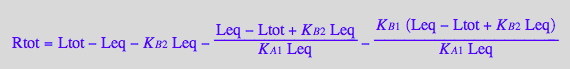
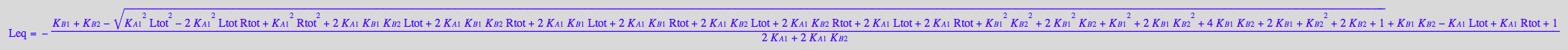
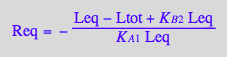
![]()
![]()
![]()
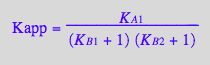
Save all numeric solutions:
filename:=CurrentPath.ProjectName.".mb";
write(filename,
// save basic equations
Rtot_U_R_L,
Leq_U_R_L,
Req_U_R_L,
Lstareq_U_R_L,
Rstareq_U_R_L,
RLeq_U_R_L,
Kapp_U_R_L,
// save functions
fLeq_U_R_L,
fReq_U_R_L,
fLstareq_U_R_L,
fRstareq_U_R_L,
fRLeq_U_R_L
)
![]()
Conclusions
1. I derived analytical solutions for the U_R_L system
2. I also derived Rtot=f([L]) for numerical solution in Matlab
3. I will do test calculations in U_R_L_analysis.mn