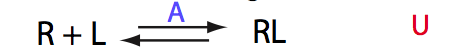
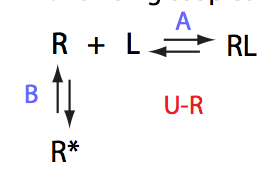
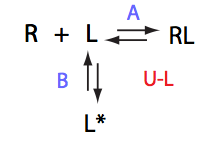
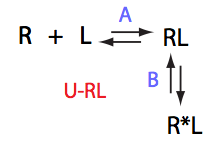
Simulate setup U_RL_Af_Bs
















Both forward and reverse titrations will have the same signature of shifting disappearing peaks and the peaks appearing at fixed position, corresponding to more populated R*L.
IMPORTANT: This spectral appearance is very similar to U_R2 model! Reverse and direct titration in that case will be different !