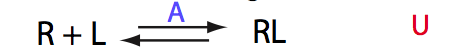
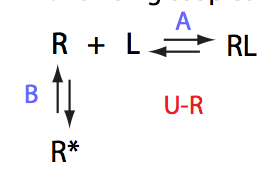
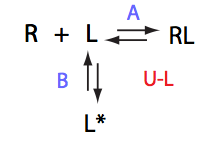
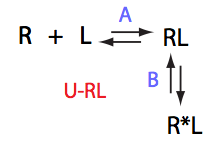
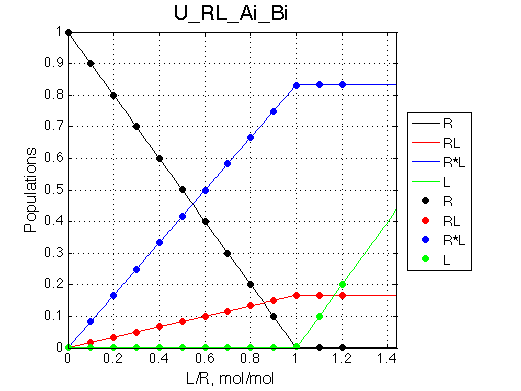
Simulate setup U_RL_Ai_Bi

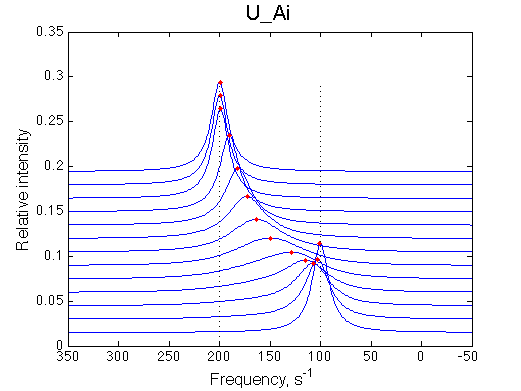
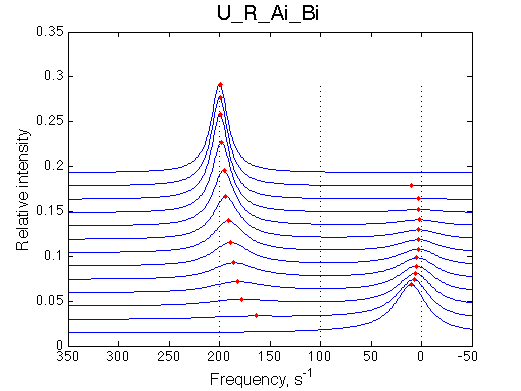
The initial peak is going into slower exchange mode as the weighted average final peak shifts away. Therefore the initial peak shifts onto the position of pure R*.
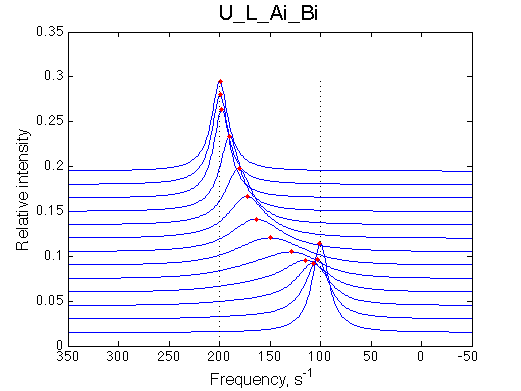
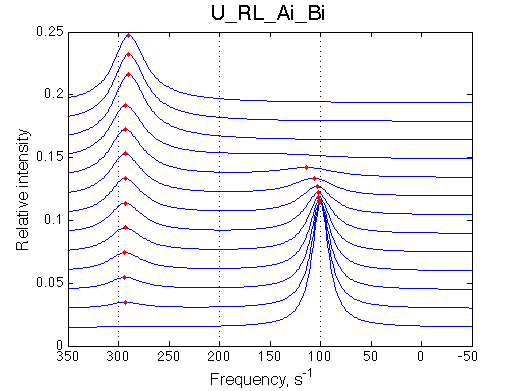
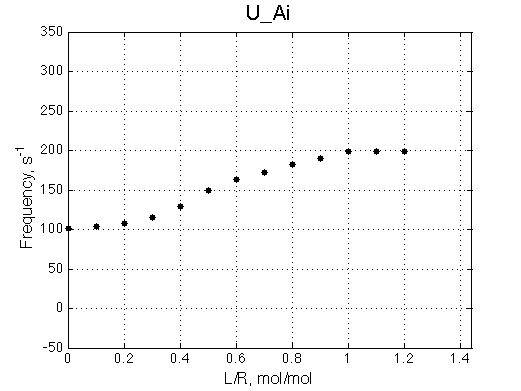
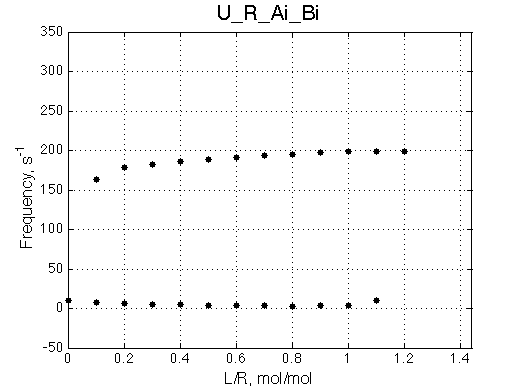
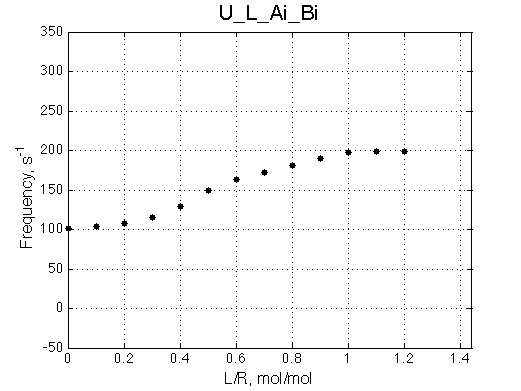
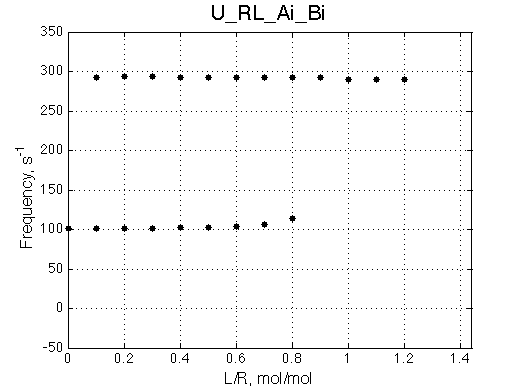
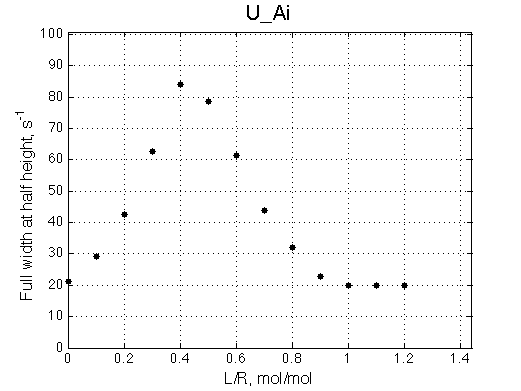
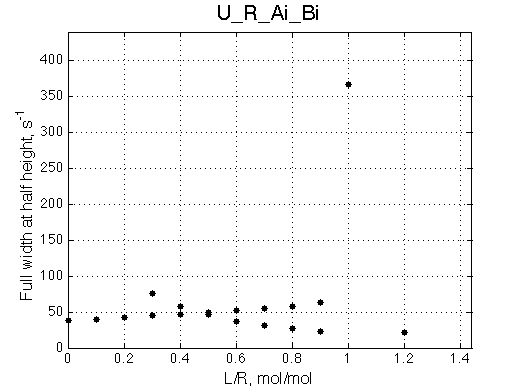
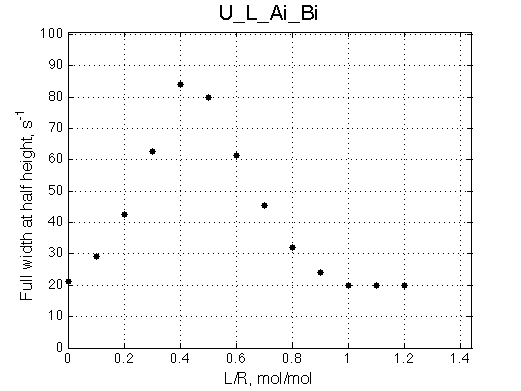
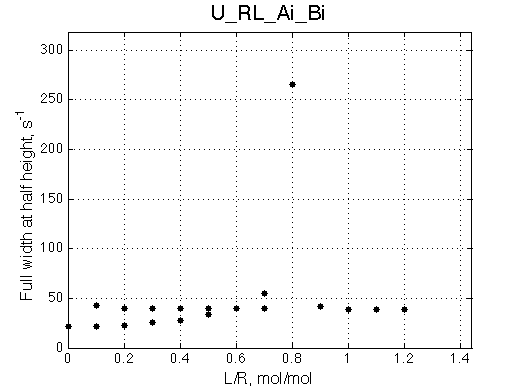
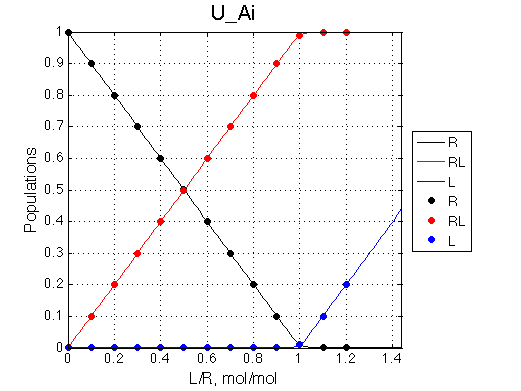
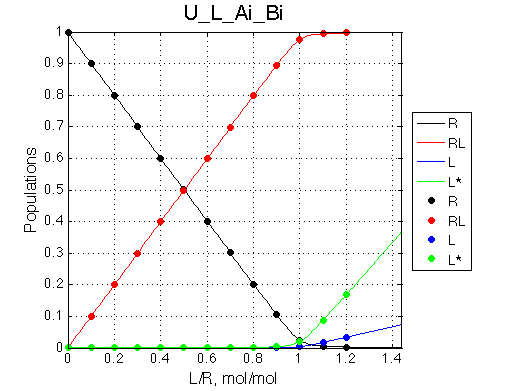
Here we will compare outcomes of the four alternative models for conditions when binding is moderately tight and the off-rate is intermediate. The isomerization is chosen to be intermediate here. I choose binding affinity of 107 M-1 and the off-rate constant of 50 s-1. R and RL are separated by 100 s-1 and R* or R*L - by 100 s-1 from corresponding species. Isomerization equilibrium is shifted 5:1 toward *-forms. The reverse isomerization rate is 17 s-1 to make kex =100 s-1 .
Location: Ai_Bi/
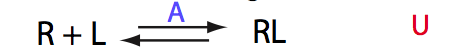 |
 |
 |
 |
Simulate setup U_RL_Ai_Bi |
|||
 |
 |
 |
 |
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| Both direct and reverse titration with give the same result. | Direct and reverse titrations will produce markedly different behavior: fast exchange for the non-isomerizing NMR-labeled component and shifting of the final and (to lower extent) initial peak | Both forward and reverse titrations will exhibit shifting initial and immobile final peaks. | |
Back to Analysis of Direct and Reverse Titrations