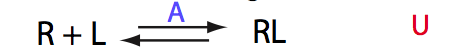
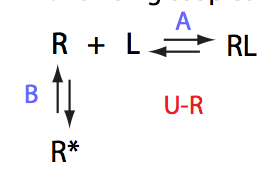
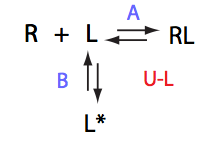
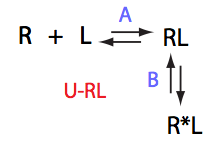
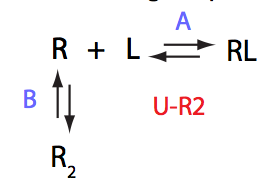
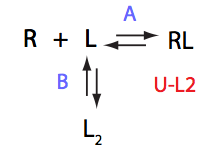
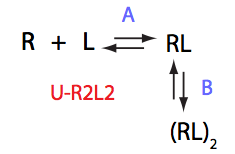
Analysis of all three-site models (U_R, U_L, U_RL, U_R2, U_L2, U_R2L2) revealed that there is a limited number of spectral appearances generated by the model. In general case every titration spectral series may be proposed to be due to at least two alternative mechanisms. However, despite the similar visual appearance of the spectra the quantitative features of the line shapes are, in many cases, different enough to tell the models apart. In other cases, measurements of concentration dependence of peak positions combined with the forward and a reverse titration are capable of unambiguously identify the undelying mechanisms. In completely ambiguous cases, the additional experiments such as ITC, relaxation dispersion or stopped-flow measurements may resolve the ambiguity.
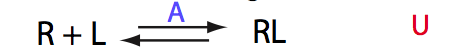 |
||
 |
 |
 |
 |
 |
 |
In this section I summarize my observations from systematic study of the model behaviors. I will make a list of spectral patterns and list all the models in all conditions I saw this pattern occuring. The list is not considered to be exhaustive, however, I hope to pinpoint most of the major patterns which may be seen in NMR spectra of the 3-site systems.
I do NOT consider possible models such as U-R if R* has low population so the coupled transition is NOT significantly operative. In this case, this will be a U model.
Another scenario is if the chemical shift difference of the coupled or binding transition is close to zero. I will only consider here cases where both transitions have substantial dw. The variation of dw may then be envisioned and modelled.
1. Slow exchange between two peaks |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
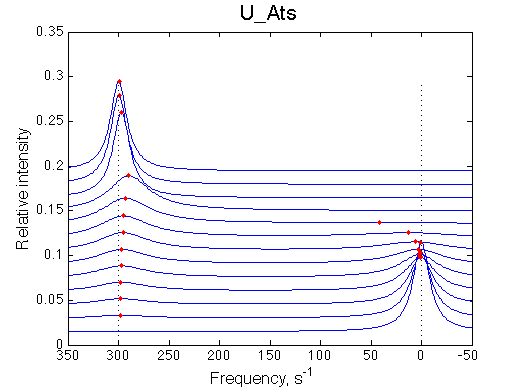 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2. Fast exchange between the two peaks |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Additional models: if the other peak is NOT identified (overlapped/unassigned) and equilibrium in isomerization/dimerization is NOT strongly shifted to one side.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
More peaks disappear than appear |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
In the crowded spectrum a small peak of another residue may be mistaken for a minor conformer: if the final peak of that another residue is exchange-broadened and NOT detected/assigned. One needs a proof of the minor peak being a minor conformer for a residue in question via triple-resonance experiments (say, HNCO/HN(CA)CO).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
More peaks appear than disappear |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
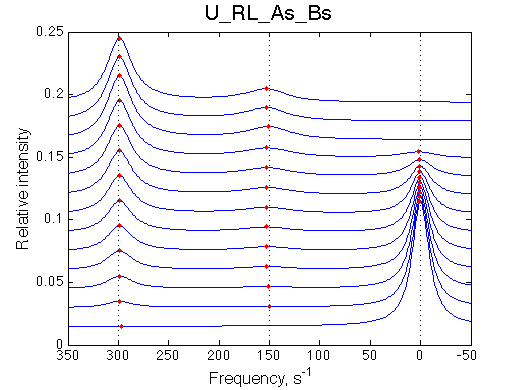 |
In the crowded spectrum a small peak of another residue may be mistaken for a minor conformer: if the initial peak of that another residue is exchange-broadened and NOT detected/assigned. One needs a proof of the minor peak being a minor conformer for a residue in question via triple-resonance experiments (say, HNCO/HN(CA)CO).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The disappearing peak is stationary while the appearing peak is moving |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
NOTE: when modeling Af regimes make sure to include both orientations of chemical shifts (crossing and non-crossing the chemical shift of end species) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The disappearing peak is moving while the appearing peak is stationary |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
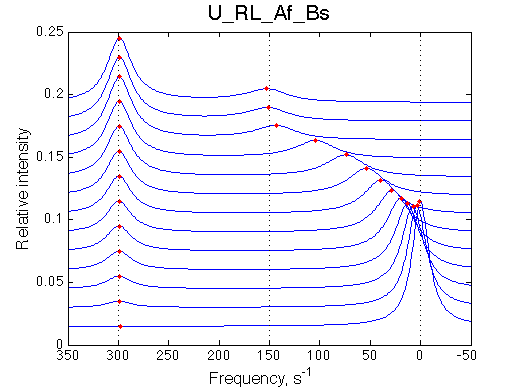 |
NOTE: when modeling Af regimes make sure to include both orientations of chemical shifts (crossing and non-crossing the chemical shift of end species) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Transient narrowing of the peak before the full saturation is achieved |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
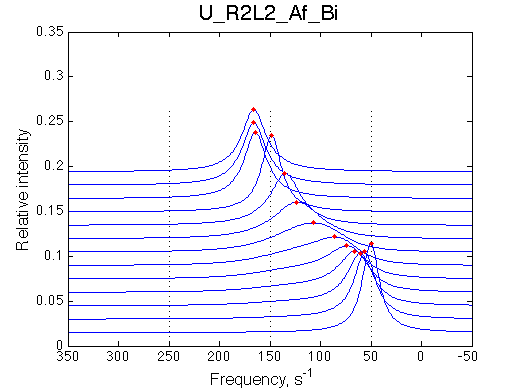 |
Conditions to observe this behavior: 1. The chemical shift of a dimer or isomer must be in between the free and bound species. 2. The equilbirium in isomerization/dimerization must be significantly shifted towards the isomer/dimer. NOTE: when modeling Af regimes make sure to include both orientations of chemical shifts (crossing and non-crossing the chemical shift of end species) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Transient narrowing of the peak at the low receptor saturation |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
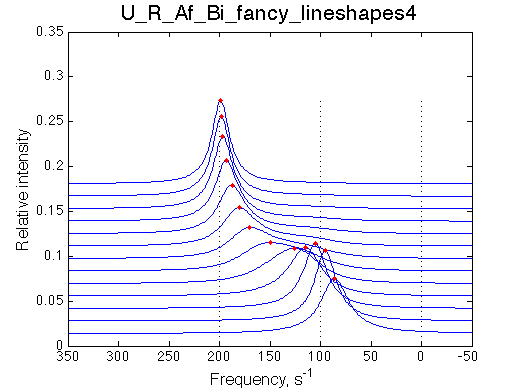 |
Conditions to observe this behavior: 1. The chemical shift of a dimer or isomer must be in between the free and bound species. 2. The equilbirium in isomerization/dimerization must be significantly shifted towards the isomer/dimer. NOTE: when modeling Af regimes make sure to include both orientations of chemical shifts (crossing and non-crossing the chemical shift of end species) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Perform three experiments:
This data will provide enough evidence for selecting the appropriate mechanism. All data together may be fit by a LineShapeKin Analysis 4 NMR line shape analysis package.
Analysis is unfinished
Back to LineShapeKin Simulation Tutorial